Solving the Problems of Plastics Adhesion
By Scott Sabreen
Problems arising from polymeric adhesion bonding are pervasive throughout the plastics industry.
Two-dimensional and three dimensional products often require bonding plastic-to-plastic, plastic-to-metal, plastic-to-composite, and so on. In addition to adhesives (epoxies, urethanes, acrylics, silicones, etc.), adhesion bonding applications include adhesion of printing inks, paints and coatings, encapsulants, potting compounds, metallization, and more. Plastic substrates are difficult to bond because they are hydrophobic (not naturally wettable); possess poor surface wettability (i.e., low surface energies); are nonpolar, inert structures; and possess poor surface-chemical functionality. This article discusses the basic science—contact angles, surface wetting, and chemical activation—behind achieving strong adhesion bond strength.
The Contact Angle
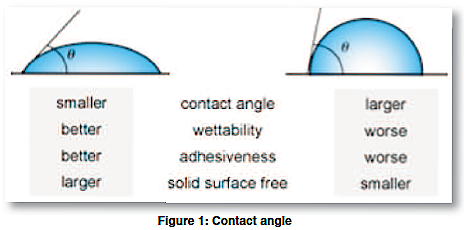
Consider a single liquid fluid droplet on a flat solid surface at rest (equilibrium state). A cross-sectional view is represented in Figure 1. The angle formed by the solid surface and the tangent line to the upper surface at the end point is called the contact angle; it is the angle between the tangent line at the contact point and the horizontal line of the solid surface. Understanding the contact angle and its physical properties of interaction between solids and liquids provides valuable information in determining optimal adhesion bonding surface wettability conditions.
The bubble/droplet shape is due to the molecular forces by which all liquids, through contraction of the surface, tend to form the contained volume into a shape having the least surface area. The intermolecular forces that contract the surface are termed “surface tension.” Surface tension, a measurement of surface energy, is expressed in “dynes/cm” (or mN/m SI units).
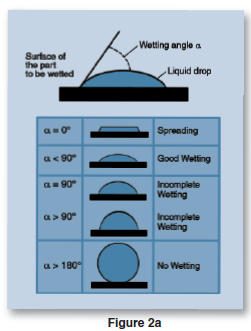
The higher the surface energy of the solid substrate relative to the surface tension of a liquid (water, printing inks, adhesives/encapsulation, coatings, etc.), the better will be its “wettability,” and the smaller will be the contact angle (Figure 2a). As a general rule, acceptable bonding adhesion is achieved when the surface energy of a substrate (measured in dynes/cm) is approximately 8 to 10 dynes/cm greater than the surface tension of the liquid. In Figure 2b, smaller contact angles are evident on the treated surfaces. Surface wetting testing involves measuring the contact angle. When a liquid does not completely wet a substrate (i.e., polymeric product), a contact angle is formed. The contact angle is a quantitative measure of the wetting of a solid by a liquid. It is a direct measure of interactions taking place between the participating phases (gas/liquid/solid or liquid/liquid/solid). The shape of the drop and the magnitude of the contact angle are controlled by the three interaction forces of interfacial tension of each participating phase (gas, liquid, solid).
For many applications it may be necessary to examine only the static equilibrium contact angle using dyne solutions in accordance with a documented test procedure such as ASTM D2578. Application kits or “dyne pens/solutions” provide useful information, but they are not precise measurements of surface tension. Surface tension measurements can vary considerably by individual (technique) and the interpretation of the “center” liquid behavior. Dyne pens/solutions are known to be directional indicators of significant differences in the surface tension and capable of identifying “good” and “bad” bondable surfaces at economical pricing.
Testing the fluid behavior of only the static contact angle can lead to misinterpretation of the liquid/solid interface results and the non-resolution of bonding problems. This is because industrial manufacturing production operations are more realistically dynamic conditions, not static. Thus the dynamic contact angle (DCA) is most important to understand.
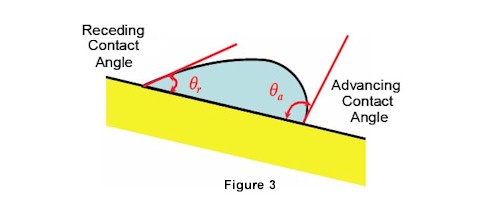
When a droplet is attached to a solid surface and the solid surface is tilted, the droplet will lunge forward and slide downward. The angles formed are termed, respectively, the advancing angle (θ a) and the receding angle (θ r). ASTM D724 describes methods for measuring DCA using advanced equipment (optical tensiometers and goniometers) to analyze advancing and receding contact angles based on drop shape analysis and mass.
Contact angles are generally considered to be affected by both changes in surface chemistry and changes in surface topography. The advancing contact angle is most sensitive to the low-energy (unmodified) components of the substrate surface, while the receding angle is more sensitive to the high-energy, oxidized groups introduced by surface pretreatments. Thus, the receding angle is actually the measurement most characteristic of the modified component of the surface following pretreatments, as measured using dyne solutions. Therefore it is important to measure both the advancing and receding contact angles on all surface-modified materials. See Figure 3.
Chemical Surface Activation
There is a strong tendency for manufacturers to focus only on contact angle measurements as the sole predictor for determining bonding problems, and conducting routine surface testing. Equally important is chemical surface functionality, by which hydrophobic surfaces are activated into bondable hydrophilic surfaces. Gas-phase, “glow-discharge,” surface oxidation pretreatment processes are used for chemical surface activation.
Gas-phase surface oxidation process methods include electrical corona discharge, flame treatment, cold gas plasma, and ultraviolet irradiation. Each method is application-specific and possesses unique advantages and potential limitations. The basic chemical and physical reaction that occurs in free electrons, ions, metastables, radicals, and UV generated in the plasma can impact a surface with energies sufficient to break the molecular bonds on the surfaces of most polymeric substrates. This creates very reactive free radicals on the polymer surface, which in turn can form, crosslink, or, in the presence of oxygen, react rapidly to form various chemical functional groups on the substrate surface. Polar functional groups that can form and enhance bondability include carbonyl (C=O), carboxyl HOOC), hydroperoxide (HOO-), and hydroxyl (HO-) groups. Even small amounts of reactive functional groups incorporated into polymers can be highly beneficial for improving surface chemical functionality and wettability. Also, chemical primers/solvents and mechanical abrasion (including mold tool texture) can be utilized alone or in conjunction with gas-phase pretreatments.2
Polymeric bonding problems are widespread and not limited to adhesives. Significant cost implications are associated with bonding failure, including poor product field performance, scrap/rework, production inefficiencies, and increased quality control inspection. Through understanding the basic science of contact angles, surface wetting, and chemical activation, processors can successfully solve virtually any bonding problem, even when using the most tough-to-bond polymeric and elastomeric materials.
References
1. Mark Strobel, Christopher Lyons, 3M Company (1995).
2. Scott R. Sabreen, The Sabreen Group Inc.,“Surface Wetting Pretreatment Methods,” Plastics Decorating (2002).
Scott R. Sabreen is founder and president of The Sabreen Group, Inc., a global engineering consulting company specializing in secondary plastics manufacturing processes: surface pretreatments, adhesion bonding, decorating and finishing, laser marking, and product security. www.sabreen.com; www.plasticslasermarking.com.