Surface Wetting and Pretreatment Methods
By Scott Sabreen, The Sabreen Group
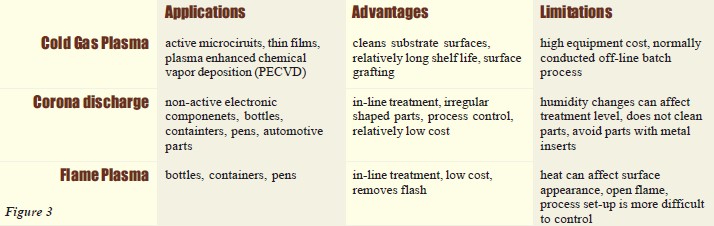
Plastics adhesion problems are widespread throughout the industry. A major contributing factor to these problems is that many plastics have chemically inert and nonporous surfaces with low surface tensions. That is, most plastics are hydrophobic and are not naturally wettable. These properties, although advantageous to the design engineer, often result in secondary assembly and decorating concerns in the areas of bonding, printing, coating and painting. However, surface pretreatments on today’s high performance engineering resins will solve many adhesion problems while increasing bond strength performance. As a general rule, acceptable bonding adhesion is achieved when the surface energy of a substrate (measured in dynes/cm) is approximately 10 dynes/cm greater than the surface tension of the liquid. In this situation, the liquid is said to “wet out” or adhere to the surface. Surface tension, which is a measurement of surface energy, is the property (due to molecular forces) by which all liquids through contraction of the surface tend to bring the contained volume onto a shape having the least surface area. Therefore, the higher the surface energy of the solid substrate relative tothe surface tension of a liquid, the better will be its “wettability”, and the smaller the contact angle (Figure 1).
As a general rule, acceptable bonding adhesion is achieved when the surface energy of a substrate (measured in dynes/cm) is approximately 10 dynes/cm greater than the surface tension of the liquid. In this situation, the liquid is said to “wet out” or adhere to the surface. Surface tension, which is a measurement of surface energy, is the property (due to molecular forces) by which all liquids through contraction of the surface tend to bring the contained volume onto a shape having the least surface area. Therefore, the higher the surface energy of the solid substrate relative tothe surface tension of a liquid, the better will be its “wettability”, and the smaller the contact angle (Figure 1).
 As many plastics are hydrophobic and are not naturally “wettable”, the table in Figure 2 lists typical surface energies for untreated polymer substrates. Due to the vast differences between resin formulations, the values listed below should be used for general benchmark purposes.
As many plastics are hydrophobic and are not naturally “wettable”, the table in Figure 2 lists typical surface energies for untreated polymer substrates. Due to the vast differences between resin formulations, the values listed below should be used for general benchmark purposes.
Pretreatment Procedures
The degree or quality of treatment is affected by the cleanliness of the plastic surface. The surface must be clean to achieve optimal pretreatment and subsequent adhesion. Surface contamination such as silicone mold release, dirt, dust, grease, oils, and fingerprints inhibit treatment. Material purity is also an important factor. The shelf life of treated plastics depends on the type of resin, formulation, and the ambient environment of the storage area. Shelf life of treated products is limited by the presence of low molecular weight oxidized materials (LMWOM) such as antioxidants, plasticizers, slip and antistatic agents, colorants and pigments, and stabilizers, etc. Exposure of treated surfaces to elevated temperatures increases molecular chain mobility – the higher the chain mobility the faster the aging of the treatment. Polymer chain mobility in treated materials causes the bonding sites created by the treatment to move away from the surface. These components may eventually migrate to the polymer surface. Therefore, it is recommended to bond, coat, paint, or decorate the product as soon as possible after pretreatment.
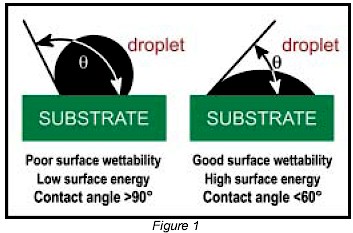
Surface pretreatments are used to increase surface energy and improve the wetting and adhesive properties of polymer materials. A variety of gas-phase surface oxidation pretreatment processes are used in the industry including low pressure cold gas plasma (Microwave/RF), electrical (corona discharge), and flame plasma. Each method is application- specific and possesses unique advantages and potential limitations (Figure 3). All of these processes are characterized by their ability to generate a “gas plasma” – an extremely reactive gas consisting of free electrons, positive ions, and other chemical species. In the science of physics, the mechanisms in which these plasmas are generated are different but their effects on surface wettability are similar. Plasmas can be conceptualized as a fourth state of matter. If sufficient energy is supplied, solids melt into liquids, liquids vaporize into gases, and gases ionize into plasmas.
Free electrons, ions, metastables, radicals, and UV generated in plasma regions can impact a surface with energies sufficient to break the molecular bonds on the surface of most substrates. This creates very reactive free radicals, on the polymer surface which in turn can form, cross-link, or in the presence of oxygen, react rapidly to form various chemical functional groups on the substrate surface. Polar functional groups that can form and enhance bondability include carbonyl (C=O), carboxyl (HOOC), hydroperoxide (HOO-), and hydroxyl (HO-) groups. Even small amounts of reactive functional groups incorporated into polymers can be highly beneficial to improving surface characteristics and wettability.
The global need to achieve robust adhesive bonding on plastics demands state-of-the-art surface pretreatment systems capable of achieving high volume production under a broad range of manufacturing applications. Several pretreatment options exist. Each project application is unique. The degree (effectiveness) of treatment on a surface is affected by prior operations such as molding and parts handling. Pretreated oxidized surfaces can affect downstream assembly process. Successful implementation (Six Sigma) is dependent upon careful evaluation of all process factors and thorough understanding of each pretreatment method.
Scott R. Sabreen is Founder & President of The Sabreen Group (TSG).TSG is an engineering company specializing in Secondary Plastics Manufacturing Operations — surface pretreatments, bonding, decorating & finishing and laser marking. For more information visit: www.sabreen.com or www.plasticslasermarking.com